| Author: | Deborah A. Marshalla,∗, Xiaoxiao Liua, Rizwan Shahidb,c, Stefania Bertazzonb,d, Judy E. Seidela,c, Alka B. Patela,c, Mina Nasre, Claire E.H. Barbera,f, Terrence McDonaldg, Rajrishi Sharmah, Tom Briggsi, Peter Farisa,j, Nigel Watersb |
| Category: | Research Papers |
| Date: | January 22, 2020 |
| Author: | Deborah A Marshall ,1,2,3,4 Xiaoxiao Liu,1,3,4 Cheryl Barnabe,1,2 Karen Yee,5 Peter D Faris,5 Claire Barber,1,2 Dianne Mosher,2 Thomas Noseworthy,1 Jason Werle,6 Lisa Lix7 |
| Category: | Research Papers |
| Date: | January 22, 2020 |
Download
Authors: Lisa Lix, James Ayles, Sharon Bartholomew, Charmaine Cooke, Joellyn Ellison, Valerie Emond, Naomi Hamm, Heather Hannah, Sonia Jean, Shannon LeBlanc, J. Michael Paterson, Catherine Pelletier, Karen Phillips, Rolf Puchtinger, Kim Reimer, Cynthia Robitaille, Mark Smith, Lawrence Svenson, Karen Tu, Linda VanTil, Sean Waits, Louise Pelletier [Canadian Chronic Disease Surveillance System (CCDSS)]
The Canadian Chronic Disease Surveillance System (CCDSS) uses administrative data to estimate prevalence of multiple chronic diseases, including arthritis.
Chronic diseases have a major impact on populations and healthcare systems worldwide. Administrative health data are an ideal resource for chronic disease surveillance because they are population-based and routinely collected. For multi-jurisdictional surveillance, a distributed model is advantageous because it does not require individual-level data to be shared across jurisdictional boundaries. Our objective is to describe the process, structure, benefits, and challenges of a distributed model for chronic disease surveillance across all Canadian provinces and territories (P/Ts) using linked administrative data.
| Category: | Research Papers |
| Date: | October 15, 2018 |
Download
Abstract
Purpose of Review
One justification for using expensive biologic therapy in rheumatoid arthritis (RA) has been that it can reduce future healthcare utilization such as joint surgeries and physician visits. However, the evidence to support this assertion is unclear. We conducted a review of the literature for studies which have analyzed the trends in resource use of RA patients, and then undertook a retrospective observational analysis of a Canadian administrative database using instrumental variable methods.
Recent Findings
Our review found a trend in reduced resource utilization prior to the introduction of biologics and no evidence that biologic therapies have specifically contributed to this reduction. Our observational analysis, which overcame some of the epidemiological challenges with determining the influence of biologics on resource utilization, found a possible reduction in other medications but possible increases rather than decreases in physician visits and hospitalizations. However, our sample was not sufficiently large to make definitive conclusions.
Summary
Over 15 years since the introduction of biologics for RA, no evidence exists supporting the assumption that biologic therapies reduce future healthcare utilization. While such a question is challenging to generate evidence for, and so an absence of evidence does not suggest that the hypothesis is incorrect, an instrumental variable analysis using sufficient data could provide definitive evidence.
| Author: | Bansback N, Fu E, Sun H, Guh D, Zhang W, Lacaille D, Milbers K, Anis AH |
| Category: | Research Papers |
| Date: | August 14, 2017 |
Download
The advent and increased use of targeted therapies in rheumatology have stimulated the establishment of clinical drug registers. Such registers have evaluated a broad spectrum of outcomes in patients exposed to these uniquely designed, potent and expensive drugs.1–8 Although the main focus of most drug registers in rheumatology is drug safety, other important issues include drug usage, real-life effectiveness and economic consequences.
| Author: | Jakub Zavada, William G Dixon, Johan Askling and for the EULAR Study group on Longitudinal Observational Registers and Drug Studies |
| Category: | Research Papers |
| Date: | December 14, 2015 |
Download
ABSTRACT
Objective: Measuring the incidence of healthcare associated infections (HAI) is of increasing importance in current healthcare delivery systems.
Administrative data algorithms, including (combinations of ) diagnosis codes, are commonly used to determine the occurrence of HAI, either to support within-hospital surveillance programmes or as free-standing quality indicators. We conducted a systematic review evaluating the diagnostic accuracy of administrative data for the detection of HAI.
Methods: Systematic search of Medline, Embase, CINAHL and Cochrane for relevant studies (1995–2013). Methodological quality assessment was performed using QUADAS-2 criteria; diagnostic accuracy estimates were stratified by HAI type and key study characteristics.
Results: 57 studies were included, the majority aiming to detect surgical site or bloodstream infections. Study designs were very diverse regarding the specification of their administrative data algorithm (code selections, follow-up) and definitions of HAI
presence. One-third of studies had important methodological limitations including differential or incomplete HAI ascertainment or lack of blinding of assessors. Observed sensitivity and positive predictive values of administrative data algorithms for HAI detection were very heterogeneous and generally modest at best, both for within-hospital algorithms and for formal quality indicators; accuracy was particularly poor for the identification of device-associated HAI such as central line associated bloodstream infections. The large heterogeneity in study designs across the included studies precluded formal calculation of summary diagnostic accuracy estimates in most instances.
Conclusions: Administrative data had limited and highly variable accuracy for the detection of HAI, and their judicious use for internal surveillance efforts and external quality assessment is recommended. If hospitals and policymakers choose to rely on administrative data for HAI surveillance, continued improvements to existing algorithms and their robust validation are imperative.
| Author: | Maaike S M van Mourik, Pleun Joppe van Duijn, Karel G M Moons, Marc J M Bonten, Grace M Lee |
| Category: | Research Papers |
| Date: | November 2, 2015 |
Download
Routinely collected health data, obtained for administrative and clinical purposes without specific a priori research goals, are increasingly used for research. The rapid evolution and availability of these data have revealed issues not addressed by existing reporting guidelines, such as Strengthening the Reporting of Observational Studies in Epidemiology (STROBE). The REporting of studies Conducted using Observational Routinely collected health Data (RECORD) statement was created to fill these gaps. RECORD was created as an extension to the STROBE statement to address reporting items specific to observational studies using routinely collected health data. RECORD consists of a checklist of 13 items related to the title, abstract, introduction, methods, results, and discussion section of articles, and other information required for inclusion in such research reports. This document contains the checklist and explanatory and elaboration information to enhance the use of the checklist. Examples of good reporting for each RECORD checklist item are also included herein. This document, as well as the accompanying website and message board (http://www.record-statement.org), will enhance the implementation and understanding of RECORD. Through implementation of RECORD, authors, journals editors, and peer reviewers can encourage transparency of research reporting.
| Author: | Eric I. Benchimol, Liam Smeeth, Astrid Guttmann, Katie Harron, David Moher, Irene Petersen, Henrik T. Sørensen, Erik von Elm, Sinéad M. Langan, RECORD Working Committee |
| Category: | Research Papers |
| Date: | November 2, 2015 |
Download
Purpose: Currently, there are no reliable and validated tools that health service decision-makers can use to inform system level policy decisions. To address this need, we worked with health administrators, clinicians and researchers to create and validate a decision-support tool that service planners can use to achieve a sustainable, integrated care system for hip and knee osteoarthritis (OA).
Methods: The tool is based on a system dynamics (SD) model of patient flow across the continuum of care, including self-directed, primary, rheumatologic and orthopaedic specialist, acute, and surgical follow-up care. The model was developed in four phases: phase 1 focused on demand and flow rates, phase 2 on resource use and costs, phase 3 on geographical stratification, and phase 4 on adding feedback loops. We populated the model with data from several sources, including Alberta Health & Wellness (e.g. physician claims, inpatient, and ambulatory data), Statistics Canada (e.g. the Survey of Living with Chronic Diseases in Canada and population projections), and the Alberta Bone and Joint Health Institute (clinical/surgical data). Using established principles of SD modeling and an iterative, integrated knowledge translation process involving multiple workshops with front-line clinical staff and administrators, we defined the problem, determined the care process, modeled the system as a series of stock and flows, tested, validated and calibrated the model.
Results: We have developed the full SD model, for two key applications. First, it can help identify flow, resource use and cost variations in current practice, which may benefit from further exploration. For example, variations in practice patterns, particularly surgery rates and resource use, were observed among the health zones reflecting regional differences. Second, it can be used to explore the effects of various ‘what if’ scenarios that can demonstrate system wide and long-term effects that may result from changes in care processes. For example, two scenarios examined were: “What would happen if 1) primary care providers could manage more patients medically, ultimately referring fewer patients to specialists; and 2) primary care providers in all health zones adopted one zone's rheumatologist referral patterns for OA patients?” Such scenarios change the pathways through which simulated patients flow, the results of which can provide insight into intended and unintended effects on resource use and costs across the continuum of care over a lengthy time horizon.
Conclusions: Our SD model can be used as a decision-support tool to estimate changes in health care demands, resource requirements and costs over time and as a result of ‘what if’ scenarios. It is critically important to involve clinicians and decision-makers in the development of such tools to ensure they are appropriate representations of the system and to facilitate their adoption and continued use to inform decision making.
| Author: | D A Marshall, S Vanderby, C Frank, E Enns, N Kulin, T Wasyluk, D Mosher, T Noseworthy, P Rogers, C Maxwell, T Rohleder, K Pkyerman, M Carter |
| Category: | Research Papers |
| Date: | November 2, 2015 |
Download
Purpose: The prevalence of osteoarthritis (OA) is increasing with the aging population; correspondingly, the demand for OA care, including hip and knee replacement surgery, is increasing. Simultaneously, combined with patients seeking surgery at younger ages and more revision surgeries, there is an increasing burden on the healthcare system. In systems with limited surgical capacity, such as Canada’s, this is raising concerns about lengthy surgical wait times. Policy makers are being called upon to identify means of managing these anticipated future demands in order to meet benchmark targets in a way that is sustainable.
| Author: | D A Marshall, S Vanderby, C Frank, T Wasylak, D Mosher, T Noseworthy, C Maxwell, K V MacDonal, M Carter |
| Category: | Research Papers |
| Date: | October 27, 2015 |
Abstract
Background
The time between symptom onset and physician diagnosis is a period when people with osteoarthritis can make lifestyle changes to reduce pain, improve function and delay disability.
Data and methods
This study analyses data for a nationally representative sample of 4,565 Canadians aged 20 or older who responded to the Arthritis component of the 2009 Survey on Living with Chronic Diseases in Canada. Descriptive statistics are used to report the prevalence of hip and knee osteoarthritis; the mean age of symptom onset and diagnosis; medication use; and contacts with health professionals during the previous year.
Results
Among people with a physician diagnosis of arthritis, 37% reported osteoarthritis. Of these, 70% experienced pain in the hip(s), knee(s), or hip(s) and knee(s). Close to half (48%) of these people experienced symptoms the same year they were diagnosed; 42% experienced symptoms at least a year before the diagnosis; and 10% experienced
symptoms after the diagnosis. Among those who had symptoms before diagnosis, the average time between symptom onset and diagnosis was 7.7 years.
Interpretation
Individuals with osteoarthritis may experience symptoms for several years before they obtain a physician diagnosis.
| Author: | Karen V MacDonald, Claudia Sanmartin, Kellie Langlois, Deborah A Marshall |
| Category: | Research Papers |
| Date: | October 27, 2015 |
Download
Estimating how many patients will require care, the nature of the care they require, and when and where they will require it, is critical when planning resources for a sustainable health-care system. Resource planning must consider how quickly patients move among stages of care, the various different pathways they may take and the resources required at each stage. This research presents a preliminary long-term, population-driven system dynamics simulation developed to support resource planning and policy development relating to osteoarthritis care. The simulation models osteoarthritis patients as they transition through the continuum of care from disease onset through end-stage care, and provides insight into the size and characteristics of the patient population, their resource requirements and associated health-care costs. Although the model presented is specific to the osteoarthritis care system in the Province of Alberta, Canada, similar methods could be applied to develop simulations relating to other chronic conditions.
| Author: | SA Vanderby, MW Carter, T Noseworthy, DA Marshall |
| Category: | Research Papers |
| Date: | October 15, 2015 |
Population attributable risk (PAR) or etiologic fraction is the proportion of a disease that could be prevented by elimination of a causal risk factor from the population. PAR is likely to be a function of time because both the prevalence of a risk factor and its effect on exposed individuals may change over time, as may the underlying risk of disease. In cohort studies with a wide range of follow-up, it may be important to account for this time dependency. Estimating the full PAR curve on some time scale of interest (e.g. age or time on study) can be more informative than considering a single value. Time-specific PAR can be estimated based on cumulative incidence adjusted for the competing risk of death. Cox models with time-dependent covariates can be used to obtain PAR estimates adjusted for confounders. The unique value of this approach is illustrated with examples that arose from our studies of heart failure in rheumatoid arthritis.
| Author: | Cynthia S Crowson, Terry M Therneau, W Michael O'Fallon |
| Category: | Research Papers |
| Date: | October 15, 2015 |
Download
Although rheumatology has been on the cutting edge of health services research for decades, there are many unresolved issues for patients, clinicians, insurers, and policy makers. This article explore three areas in which methodologic controversies present tradeoffs to a health care system that is grappling with larger issues around cost and access to care. Specifically, we examine issues around the use of large databases, the appropriate instruments for measuring patient-centered outcomes, and the questions that are raised from cost effectiveness studies of new treatments for rheumatoid arthritis (RA). The issues are presented in the context of a need to provide better information to those who are providing care and those who are paying for it.
| Author: | Sheikh Usman Iqbal, Mark Prashker |
| Category: | Research Papers |
| Date: | October 6, 2015 |
Download
SUMMARY
Purpose Administrative healthcare utilization data from Canadian provinces have been used for pharmacoepidemiology and pharmacoeconomics research, but limited transparency exists about opportunities for data access, who can access them, and processes to obtain data. An attempt was made to obtain data from all 10 provinces to evaluate access and its complexity. Methods An initial enquiry about the process and requirements to obtain data on individual, anonymized patients dispensed any of four antiviral drugs in the ambulatory setting, linked with data from hospital and physician service claims, was sent to each province. Where a response was encouraging, a technical description of the data of interest was submitted. Results Data were unavailable from the provinces of New Brunswick, Newfoundland and Labrador, and Prince Edward Island, and inaccessible from British Columbia, Manitoba and Ontario due to policies that prohibit collaborative work with pharmaceutical industry researchers. In Nova Scotia, patient-level data were available but only on site. Data were accessible in Alberta, Quebec and Saskatchewan, although variation exists in the currency of the data, time to obtain data, approval requirements and insurance coverage eligibility. Conclusions As Canada moves towards a life-cycle management approach to drug regulation, more post-marketing studies will be required, potentially using administrative data. Linked patient-level drug and healthcare data are presently accessible to pharmaceutical industry researchers in four provinces, although only logistically realistic in three and limited to seniors and low-income individuals in two. Collaborative endeavours to improve access to provincial data and to create other data resources should be encouraged.
| Author: | Nigel S B Rawson |
| Category: | Research Papers |
| Date: | October 6, 2015 |
ABSTRACT
Background
In Canada, programs are being developed to supply hospital emergency departments and family doctors with electronic access to their patients’ drug history profiles. While some of these programs have access to databases that capture information about all out-patient prescriptions that are dispensed to an individual, regardless of payer; others do not, and rely upon claims paid by their provincial drug benefit plans. The completeness of these latter profiles is unknown.
Objectives
To estimate the percentage of Ontario seniors who use private drug insurance (as an indicator of the potential for a ‘public’ drug history profile to be incomplete) and to describe the kinds of medications for which private insurance is used.
Methods
Cross-sectional time series analysis of Ontario Drug Benefit (ODB) claims and private drug insurance claims for Ontario residents aged 65 years or older (seniors) covering the period January 2000 to December 2005.
Results
During the study period, approximately 95% of Ontario seniors filled at least one prescription paid by the provincial drug benefit plan. By comparison, approximately 15-20% filled a prescription paid by a private insurer. Compared to the 20 drugs most frequently subsidized by the ODB Program (all but one of which had ODB full benefit status), the top privately-purchased drugs were more diverse: 8 had ODB full benefit status; 4 had ODB Limited Use status (which requires that patients meet prespecified clinical criteria for coverage); 3 required individual clinical review (prior authorization) by the ODB Program; and 5 were ODB non-benefits.
Conclusions
Many Ontario seniors are at risk for an incomplete ODB drug history profile. Further research is needed to confirm whether this causes problems for physicians and patients.
| Author: | J Michael Paterson, Ali Suleiman, Janet E Hux, Chaim Bell |
| Category: | Research Papers |
| Date: | September 24, 2015 |
Abstract
Background: With aging and obesity trends, the incidence and prevalence of osteoarthritis (OA) are expected to rise in Canada, increasing the demand for health resources. Resource planning to meet this increasing need requires estimates of the anticipated number of OA patients. Using administrative data from Alberta, we estimated OA incidence and prevalence rates and examined their sensitivity to alternative case definitions.
Methods: We identified cases in a linked dataset spanning 1993 to 2010 (Population Registry, Discharge Abstract Database, Physician Claims, Ambulatory Care Classification System and prescription drug data) using diagnostic codes and drug identification numbers. In the base case, incident cases were captured for patients with an OA diagnostic code for at least two physician visits within two years or any hospital admission. Seven alternative case definitions were applied and compared.
Results: Age-sex standardized incidence and prevalence rates were estimated to be 8.6 and 80.3 cases/1000 population, respectively, in the base case. Physician Claims data alone captured 88% of OA cases. Prevalence rate estimates required 15 years of longitudinal data to plateau. Compared to base case, estimates are sensitive to alternative case definitions.
Conclusion: Administrative databases are a key source for estimating the burden and epidemiological trends of chronic diseases such as OA in Canada. Despite their limitations, these data provide valuable information for estimating disease burden and planning health services. Estimates of OA are mostly defined through Physician Claims data and require a long period of longitudinal data.
| Author: | Deborah A Marshall, Sonia Vanderby, Cheryl Barnabe, Karen V MacDonald, Colleen Maxwell, Dianne Mosher, Tracy Wasylak, Lisa Lix, Ed Enns, Cy Frank, Tom Noseworthy |
| Category: | Research Papers |
| Date: | September 16, 2015 |
Download
Abstract: Misclassification is present in nearly every epidemiologic study, yet is rarely quantified in analysis in favor of a focus on random error. In this review, we discuss past and present wisdom on misclassification and what measures should be taken to quantify this influential bias, with a focus on bias in pharmacoepidemiologic studies. To date, pharmacoepidemiology primarily uses data obtained from administrative claims, a rich source of prescription data, but susceptible to bias from unobservable factors including medication sample use, medications filled but not taken, health conditions that are not reported in the administrative billing data, and inadequate capture of confounders. Because of the increasing focus on comparative effectiveness research, we provide a discussion of misclassification in the context of an active comparator, including a demonstration of treatment effects biased away from the null in the presence of nondifferential misclassification. Finally, we highlight recently developed methods to quantify bias and offer these methods as potential options for strengthening the validity and quantifying uncertainty of results obtained from pharmacoepidemiologic research.
| Author: | Michele Jonsson Funk, Suzanne N Landi |
| Category: | Research Papers |
| Date: | September 16, 2015 |
Download
This manual presents the lists of causes of death for tabulation and dissemination of mortality data by the National Center for Health Statistics (NCHS) under the Tenth Revision of the International Classification of Diseases (ICD-10) recommended by the World Health Organization (WHO)(1). NCHS began using this manual for data year 1999. A total of eight (8) tabulation lists are described. Seven (7) of the lists are used for both underlying and multiple causes of death, and one for multiple causes of death only. The tabulation lists in this report have been developed to maintain continuity with the lists from the Ninth Revision of the International Classification of Diseases (ICD-9)(2); to facilitate trend analysis; and to separately identify causes of death that are of public health and medical importance. Periodically, NCHS Instruction Manuals are updated in order to correct errors and to reflect needed modifications dictated by changes in medical science, statistical practice and processing technology. This update represents the fourth to the lists that are used by NCHS to present mortality data and include the addition of ICD-10 codes for Terrorism Deaths for data year 2001 and WHO updates to ICD-10 for data year 2003. States and other federal agencies and organizations using the NCHS lists should incorporate these changes as soon as possible. All changes listed in the “Notices of Change” have been incorporated into this updated version of the manual (see Appendices A-D).
| Author: | Robert N. Anderson, James A Weed, Kenneth D Kochaneck, LloydReich, Brenda Gillum, Jeffrey D. Maurer, Linda Le |
| Category: | Consensus Statements and Best Practices |
| Date: | September 9, 2015 |
Download
Background
Linkages between registries and administrative data may provide a valuable resource for comparative effectiveness research. However, personal identifiers that uniquely identify individuals are not always available. We describe methods to link a de-identified arthritis registry and U.S. Medicare data. The linked dataset was also used to evaluate the generalizability of the registry to the U.S. Medicare population.
Methods
Rheumatoid arthritis (RA) patients participating in the Consortium of Rheumatology Researchers of North America (CORRONA) registry were linked to Medicare data restricted to rheumatology claims or claims for RA. Deterministic linkage was performed using age, sex, provider identification number, and geographic location of the CORRONA site. We then searched for visit dates in Medicare matching visit dates in CORRONA, requiring at least 1 exact matching date. Linkage accuracy was quantified as a positive predictive value (PPV) in a sub-cohort (n=1581) with more precise identifiers.
Results
CORRONA participants with self-reported Medicare (n=11,001) were initially matched to 30,943 Medicare beneficiaries treated by CORRONA physicians. A total of 8,431 CORRONA participants matched on at least 1 visit; 5,317 matched uniquely on all visits. The number of patients who linked and linkage accuracy (from the subcohort) was high for patients with >2 visits (n=3458, 98% accuracy), exactly 2 visits (n=822, 96% accuracy) visits, and 1 visit (n=1037, 79% accuracy) visit that matched exactly on calendar date. Demographics and comorbidity profiles of registry participants were similar to non-participants, except participants were more likely to use DMARDs and biologics.
Conclusion
Linkage between a national, de-identified outpatient arthritis registry and Medicare data on multiple non-unique identifiers appears feasible and valid.
| Author: | Curts, J R, Chen L, Bharat A, Delzell E, Greenberg J D, Harrold L, Kremer J, Setoguchi S, Solomon D H, Xie F, Yun H. |
| Category: | Research Papers |
| Date: | September 9, 2015 |
Download
Introduction. Routinely collected primary care data has underpinned research that has helped define primary care as a specialty. In the early years of the discipline, data were collected manually, but digital data collection now makes large volumes of data readily available. Primary care informatics is emerging as an academic discipline for the scientific study of how to harness these data. This paper reviews how data are stored in primary care computer systems; current use of large primary care research databases; and, the opportunities and challenges for using routinely collected primary care data in research. Opportunities. (1) Growing volumes of routinely recorded data. (2) Improving data quality. (3) Technological progress enabling large datasets to be processed. (4) The potential to link clinical data in family practice with other data including genetic databases. (5) An established body of know-how within the international health informatics community. Challenges. (1) Research methods for working with large primary care datasets are limited. (2) How to infer meaning from data. (3) Pace of change in medicine and technology. (4) Integrating systems where there is often no reliable unique identifier and between health (person-based records) and social care (care-based records—e.g. child protection). (5) Achieving appropriate levels of information security, confidentiality, and privacy.
Conclusion. Routinely collected primary care computer data, aggregated into large databases, is used for audit, quality improvement, health service planning, epidemiological study and research. However, gaps exist in the literature about how to find relevant data, select appropriate research methods and ensure that the correct inferences are drawn.
| Author: | Simon de Lusignan and Chris van Weel |
| Category: | Research Papers |
| Date: | September 1, 2015 |
This review assessed the use of electronic medical record (EMR) systems in outcomes research. We systematically searched PubMed to identify articles published from January 2000 to January 2007 involving EMR use for outpatient-based outcomes research in the United States. EMR-based outcomes research studies (n = 126) have increased sixfold since 2000. Although chronic conditions were most common, EMRs were also used to study less common diseases, highlighting the EMRs’ flexibility to examine large cohorts as well as identify patients with rare diseases. Traditional multivariate modeling techniques were the most commonly used technique to address confounding and potential selection bias. Data validation was a component in a quarter of studies, and many evaluated the EMR’s ability to achieve similar results previously achieved using other data sources. Investigators using EMR data should aim for consistent terminology, focus on adequately describing their methods, and consider appropriate statistical methods to control for confounding and treatment-selection bias.
| Author: | Bonnie B. Dean, Jessica Lam, Jaime L Natoli, Qiana Bulter, Daniel Aguilar, Robert J Nordyke |
| Category: | Research Papers |
| Date: | August 25, 2015 |
Abstract
Introduction: Use of disease-modifying anti-rheumatic drugs (DMARDs) in rheumatoid arthritis (RA) may prevent joint damage and potentially reduce joint replacement surgeries. We assessed the association between RA drug use and joint replacement in Quebec, Canada.
Methods: A cohort of new-onset RA patients was identified from Quebec’s physician billing and hospitalization databases from 2002–2011. The outcome was defined using procedure codes submitted by orthopedic surgeons. Medication use was obtained from pharmacy databases. We used alternative Cox regression models with
time-dependent variables measuring the cumulative effects of past use during different time windows (one model focussing on the first year after cohort entry) for methotrexate (MTX), and other DMARDs. Models were adjusted for baseline sociodemographics, co-morbidity and prior health service use, time-dependent cumulative use of other drugs (anti-tumor necrosis factor [anti-TNF] agents, other biologics, cyclooxygenase-2 inhibitors [COXIBs], nonselective nonsteroidal antiinflammatory drugs [NSAIDs], and systemic steroids), and markers of disease severity.
Results: During follow-up, 608 joint replacements occurred among 11,333 patients (median follow-up: 4.6 years). The best-fitting model relied on the cumulative early use (within the first year after cohort entry) of MTX and of other DMARDs, with an interaction between MTX and other DMARDs. In this model, greater exposure within the first year, to either MTX (adjusted hazard ratio, HR = 0.95 per 1 month, 95 % confidence interval, 95 % CI 0.93-0.97) or other DMARDs (HR = 0.97, 95 % CI 0.95-0.99) was associated with longer time to joint replacement.
Conclusions: Our results suggest that longer exposure to either methotrexate (MTX) or other DMARDs within the first year after RA diagnosis is associated with longer time to joint replacement surgery.
Authors:Cristiano S. Moura, Michal Abrahamowicz, Marie-Eve Beauchamp, Diane Lacaille, Yishu Wang, Gilles Boire, Paul R. Fortin, Louis Bessette, Claire Bombardier, Jessica Widdifield, John G. Hanly, Debbie Feldman, Walter Maksymowych, Christine Peschken, Cheryl Barnabe, Steve Edworthy, Sasha Bernatsky and CAN-AIM
| Category: | Research Papers |
| Date: | August 18, 2015 |
Download
Abstract
Objectives: To conduct a systematic review of studies reporting on the development or validation of comorbidity indices using administrative health data and compare their ability to predict outcomes related to comorbidity (ie, construct validity).
Study Design and Setting: We conducted a comprehensive literature search of MEDLINE and EMBASE, until September 2012. After title and abstract screen, relevant articles were selected for review by two independent investigators. Predictive validity and model fit were measured using c-statistic for dichotomous outcomes and R2 for continuous outcomes.
Results: Our review includes 76 articles. Two categories of comorbidity indices were identified: those identifying comorbidities based on diagnoses, using International Classification of Disease codes from hospitalization or outpatient data, and based on medications, using pharmacy data. The ability of indices studied to predict morbidity-related outcomes ranged from poor (C statistic 0.69) to excellent (C statisticO0.80) depending on the specific index, outcome measured, and study population. Diagnosis-based measures, particularly the Elixhauser Index and the Romano adaptation of the Charlson Index, resulted in higher ability to predict mortality outcomes. Medication-based indices, such as the Chronic Disease Score, demonstrated better performance for predicting health care utilization.
Conclusion: A number of valid comorbidity indices derived from administrative data are available. Selection of an appropriate index should take into account the type of data available, study population, and specific outcome of interest. 2015 Elsevier Inc. All rights reserved.
| Author: | Marko Yurkovich J. Antonio Avina-Zubieta, Jamie Thomas, Mike Gorenchtein, Diane Lacaille |
| Category: | Research Papers |
| Date: | August 18, 2015 |
Download
Abstract
Background: Though administrative databases are increasingly being used for research related to myocardial infarction (MI),
the validity of MI diagnoses in these databases has never been synthesized on a large scale.
Objective: To conduct the first systematic review of studies reporting on the validity of diagnostic codes for identifying MI
in administrative data.
Methods: MEDLINE and EMBASE were searched (inception to November 2010) for studies: (a) Using administrative data to
identify MI; or (b) Evaluating the validity of MI codes in administrative data; and (c) Reporting validation statistics (sensitivity,
specificity, positive predictive value (PPV), negative predictive value, or Kappa scores) for MI, or data sufficient for their
calculation. Additonal articles were located by handsearch (up to February 2011) of original papers. Data were extracted by
two independent reviewers; article quality was assessed using the Quality Assessment of Diagnostic Accuracy Studies tool.
Results: Thirty studies published from 1984–2010 were included; most assessed codes from the International Classification
of Diseases (ICD)-9th revision. Sensitivity and specificity of hospitalization data for identifying MI in most [$50%] studies was
$86%, and PPV in most studies was $93%. The PPV was higher in the more-recent studies, and lower when criteria that do
not incorporate cardiac troponin levels (such as the MONICA) were employed as the gold standard. MI as a cause-of-death
on death certificates also demonstrated lower accuracy, with maximum PPV of 60% (for definite MI).
Conclusions: Hospitalization data has higher validity and hence can be used to identify MI, but the accuracy of MI as a
cause-of-death on death certificates is suboptimal, and more studies are needed on the validity of ICD-10 codes. When
using administrative data for research purposes, authors should recognize these factors and avoid using vital statistics data
if hospitalization data is not available to confirm deaths from MI.
Received September 29, 2013; Accepted February 20, 2014; Published March 28, 2014
Citation: McCormick N, Lacaille D, Bhole V, Avina-Zubieta JA (2014) Validity of Myocardial Infarction Diagnoses in Administrative Databases: A Systematic
Review. PLoS ONE 9(3): e92286. doi:10.1371/journal.pone.0092286
| Author: | Natalie McCormick, Diane Lacaille, Vidula Bhole, J. Antonio Avina-Zubieta |
| Category: | Research Papers |
| Date: | July 30, 2015 |
Abstract
Introduction: Administrative health data have been used to study sepsis in large population-based studies. The
validity of these study findings depends largely on the quality of the administrative data source and the validity of
the case definition used. We systematically reviewed the literature to assess the validity of case definitions of sepsis
used with administrative data.
Methods: Embase and MEDLINE were searched for published articles with International Classification of Diseases
(ICD) coded data used to define sepsis. Abstracts and full-text articles were reviewed in duplicate. Data were
abstracted from all eligible full-text articles, including ICD-9- and/or ICD-10-based case definitions, sensitivity (Sn),
specificity (Sp), positive predictive value (PPV) and negative predictive value (NPV).
Results: Of 2,317 individual studies identified, 12 full-text articles met all eligibility criteria. A total of 38 sepsis case
definitions were tested, which included over 130 different ICD codes. The most common ICD-9 codes were 038.x,
790.7 and 995.92, and the most common ICD-10 codes were A40.x and A41.x. The PPV was reported in ten studies
and ranged from 5.6% to 100%, with a median of 50%. Other tests of diagnostic accuracy were reported only in
some studies. Sn ranged from 5.9% to 82.3%; Sp ranged from 78.3% to 100%; and NPV ranged from 62.1% to 99.7%.
Conclusions: The validity of administrative data in recording sepsis varied substantially across individual studies
and ICD definitions. Our work may serve as a reference point for consensus towards an improved and harmonized
ICD-coded definition of sepsis.
Jolley et al. Critical Care (2015) 19:139
DOI 10.1186/s13054-015-0847-3
| Author: | Rachel J Jolley, Keri Jo Sawka, Dean W Yergens, Hude Quan, Nathalie Jetté, and Christopher J Doig |
| Category: | Research Papers |
| Date: | July 30, 2015 |
Download
Abstract
Background
Prescription medication use, which is common among long-term care facility (LTCF) residents, is routinely used to describe quality of care and predict health outcomes. Data sources that capture medication information, which include surveys, medical charts, administrative health databases, and clinical assessment records, may not collect concordant information, which can result in comparable prevalence and effect size estimates. The purpose of this research was to estimate agreement between two population-based electronic data sources for measuring use of several medication classes among LTCF residents: outpatient prescription drug administrative data and the Resident Assessment Instrument Minimum Data Set (RAI-MDS) Version 2.0.
Methods
Prescription drug and RAI-MDS data from the province of Saskatchewan, Canada (population 1.1 million) were linked for 2010/11 in this cross-sectional study. Agreement for anti-psychotic, anti-depressant, and anti-anxiety/hypnotic medication classes was examined using prevalence estimates, Cohen’s κ, and positive and negative agreement. Mixed-effects logistic regression models tested resident and facility characteristics associated with disagreement.
Results
The cohort was comprised of 8,866 LTCF residents. In the RAI-MDS data, prevalence of anti-psychotics was 35.7%, while for anti-depressants it was 37.9% and for hypnotics it was 27.1%. Prevalence was similar in prescription drug data for anti-psychotics and anti-depressants, but lower for hypnotics (18.0%). Cohen’s κ ranged from 0.39 to 0.85 and was highest for the first two medication classes. Diagnosis of a mood disorder and facility affiliation was associated with disagreement for hypnotics.
Conclusions
Agreement between prescription drug administrative data and RAI-MDS assessment data was influenced by the type of medication class, as well as selected patient and facility characteristics. Researchers should carefully consider the purpose of their study, whether it is to capture medication that are dispensed or medications that are currently used by residents, when selecting a data source for research on LTCF populations.
BMC Geriatrics 2015, 15:24 (11 March 2015).
| Author: | Lix LM, Yan L, Blackburn D, Hu N, Schneider-Lindner V, Shevchuk Y, Teare GF |
| Category: | Research Papers |
| Date: | July 30, 2015 |
Abstract
Background: Erroneous patient birthdates are common in health databases. Detection of these errors usually
involves manual verification, which can be resource intensive and impractical. By identifying a frequent
manifestation of birthdate errors, this paper presents a principled and statistically driven procedure to identify
erroneous patient birthdates.
Results: Generalized additive models (GAM) enabled explicit incorporation of known demographic trends and birth
patterns. With false positive rates controlled, the method identified birthdate contamination with high accuracy.
In the health data set used, of the 58 actual incorrect birthdates manually identified by the domain expert, the
GAM-based method identified 51, with 8 false positives (resulting in a positive predictive value of 86.0% (51/59) and
a false negative rate of 12.0% (7/58)). These results outperformed linear time-series models.
Conclusions: The GAM-based method is an effective approach to identify systemic birthdate errors, a common
data quality issue in both clinical and administrative databases, with high accuracy.
BMC Bioinformatics 2014, 15:185
Published: 12 June 2014
| Author: | Wei Luo, Marcus Gallagher, Bill Loveday, Susan Ballantyne, Jason P Connor, and Janet Wiles |
| Category: | Research Papers |
| Date: | July 16, 2015 |
Download
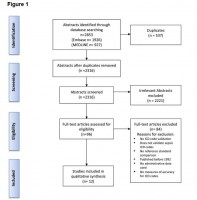
Appropriate use of administrative data enables the assessment of care quality at the population level. Our objective was to develop/validate methods for assessing quality of breast cancer diagnostic care using administrative data, specifically by identifying relevant medical tests to estimate the percentage screen/symptom-detected cancers and time to diagnosis. Two databases were created for all women diagnosed with a first-ever breast cancer in years 2007–2010 in Alberta, Canada, with dates of medical tests received in years 2006–2010. One purchased database had test results and was used to determine the ‘true’ first relevant test of a cancer diagnosis. The other free administrative database had test types but no test results. Receiver operating characteristic curves and concordance rates were used to assess estimates of percent screen/symptom-detected breast cancers; Log-rank test was used to assess time to diagnosis obtained from the two databases. Using a look-back period of 4–6 months from cancer diagnosis to identify relevant tests resulted in over 94% concordance, sensitivity and specificity for classifying patients into screen/symptom-detected group; good agreement between the distributions of time to diagnosis was also achieved. Our findings support the use of administrative data to accurately identify relevant tests for assessing the quality of breast cancer diagnostic care.
Article first published online: 17 DEC 2014
DOI: 10.1111/ecc.12277
| Author: | Yuan Y, Li M, Yang J, Winget M. |
| Category: | Research Papers |
| Date: | July 16, 2015 |
Abstract
Background: Erroneous patient birthdates are common in health databases. Detection of these errors usually involves manual verification, which can be resource intensive and impractical. By identifying a frequent manifestation of birthdate errors, this paper presents a principled and statistically driven procedure to identify erroneous patient birthdates.
Results: Generalized additive models (GAM) enabled explicit incorporation of known demographic trends and birth patterns. With false positive rates controlled, the method identified birthdate contamination with high accuracy.
In the health data set used, of the 58 actual incorrect birthdates manually identified by the domain expert, the GAM-based method identified 51, with 8 false positives (resulting in a positive predictive value of 86.0% (51/59) and a false negative rate of 12.0% (7/58)). These results outperformed linear time-series models.
Conclusions: The GAM-based method is an effective approach to identify systemic birthdate errors, a common data quality issue in both clinical and administrative databases, with high accuracy.
BMC Bioinformatics 2014, 15:185
Published: 12 June 2014
More information
| Author: | Wei Luo, Marcus Gallagher, Bill Loveday, Susan Ballantyne, Jason P Connor, and Janet Wiles |
| Category: | Research Papers |
| Date: | June 4, 2015 |
Download
Appropriate use of administrative data enables the assessment of care quality at the population level. Our objective was to develop/validate methods for assessing quality of breast cancer diagnostic care using administrative data, specifically by identifying relevant medical tests to estimate the percentage screen/symptom-detected cancers and time to diagnosis. Two databases were created for all women diagnosed with a first-ever breast cancer in years 2007–2010 in Alberta, Canada, with dates of medical tests received in years 2006–2010. One purchased database had test results and was used to determine the ‘true’ first relevant test of a cancer diagnosis. The other free administrative database had test types but no test results. Receiver operating characteristic curves and concordance rates were used to assess estimates of percent screen/symptom-detect ed breast cancers; Log-rank test was used to assess time to diagnosis obtained from the two databases. Using a look-back period of 4–6 months from cancer diagnosis to identify relevant tests resulted in over 94% concordance, sensitivity and specificity for classifying patients into screen/symptom-detected group; good agreement between the distributions of time to diagnosis was also achieved. Our findings support the use of administrative data to accurately identify relevant tests for assessing the quality of breast cancer diagnostic care.
Article first published online: 17 DEC 2014
DOI: 10.1111/ecc.12277
| Author: | Yuan Y, Li M, Yang J, Winget M. |
| Category: | Research Papers |
| Date: | June 4, 2015 |
Download
Abstract
Background
Prescription medication use, which is common among long-term care facility (LTCF) residents, is routinely used to describe quality of care and predict health outcomes. Data sources that capture medication information, which include surveys, medical charts, administrative health databases, and clinical assessment records, may not collect concordant information, which can result in comparable prevalence and effect size estimates. The purpose of this research was to estimate agreement between two population-based electronic data sources for measuring use of several medication classes among LTCF residents: outpatient prescription drug administrative data and the Resident Assessment Instrument Minimum Data Set (RAI-MDS) Version 2.0.
Methods
Prescription drug and RAI-MDS data from the province of Saskatchewan, Canada (population 1.1 million) were linked for 2010/11 in this cross-sectional study. Agreement for anti-psychotic, anti-depressant, and anti-anxiety/hypnotic medication classes was examined using prevalence estimates, Cohen’s κ, and positive and negative agreement. Mixed-effects logistic regression models tested resident and facility characteristics associated with disagreement.
Results
The cohort was comprised of 8,866 LTCF residents. In the RAI-MDS data, prevalence of anti-psychotics was 35.7%, while for anti-depressants it was 37.9% and for hypnotics it was 27.1%. Prevalence was similar in prescription drug data for anti-psychotics and anti-depressants, but lower for hypnotics (18.0%). Cohen’s κ ranged from 0.39 to 0.85 and was highest for the first two medication classes. Diagnosis of a mood disorder and facility affiliation was associated with disagreement for hypnotics.
Conclusions
Agreement between prescription drug administrative data and RAI-MDS assessment data was influenced by the type of medication class, as well as selected patient and facility characteristics. Researchers should carefully consider the purpose of their study, whether it is to capture medication that are dispensed or medications that are currently used by residents, when selecting a data source for research on LTCF populations.
BMC Geriatrics 2015, 15:24 (11 March 2015).
| Author: | Lix LM, Yan L, Blackburn D, Hu N, Schneider-Lindner V, Shevchuk Y, Teare GF |
| Category: | Research Papers |
| Date: | May 21, 2015 |
Download
Abstract
Objective
To estimate the association between fine particulate (PM2.5) and nitrogen dioxide (NO2) pollution and systemic autoimmune rheumatic diseases (SARDs).
Methods
Associations between ambient air pollution (PM2.5 and NO2) and SARDs were assessed using land-use regression models for Calgary, Alberta and administrative health data (1993–2007). SARD case definitions were based on ≥2 physician claims, or ≥1 rheumatology billing code; or ≥1 hospitalization code (for systemic lupus, Sjogren's Syndrome, scleroderma, polymyositis, dermatomyositis, or undifferentiated connective tissue disease). Bayesian hierarchical latent class regression models estimated the probability that each resident was a SARD case, based on these case definitions. The sum of individual level probabilities provided the estimated number of cases in each area. The latent class model included terms for age, sex, and an interaction term between age and sex. Bayesian logistic regression models were used to generate adjusted odds ratios (OR) for NO2 and PM2.5. pollutant models, adjusting for neighbourhood income, age, sex, and an interaction between age and sex. We also examined models stratified for First-Nations (FN) and non-FN subgroups.
Results
Residents that were female and/or aged >45 had a greater probability of being a SARD case, with the highest OR estimates for older females. Independently, the odds of being a SARDs case increased with PM2.5 levels, but the results were inconclusive for NO2. The results stratified by FN and non-FN groups were not distinctly different.
Conclusion
In this urban Canadian sample, adjusting for demographics, exposure to PM2.5 was associated with an increased risk of SARDs. The results for NO2 were inconclusive.
Environmental Research Volume 140, July 2015, Pages 474–478.
| Author: | Sasha Bernatsky, AudreySmargiassi, MarkeyJohnson, Gilaad G.Kaplan, Cheryl Barnabe, Larry Svenson, Allan Brand, Stefania Bertazzon, Marie Hudson, Ann E.Clarke, Paul R.Fortin, Steven Edworthy, Patrick Bélisle, Lawrence Joseph |
| Category: | Research Papers |
| Date: | May 20, 2015 |
Download
PUB MED Information on article. Can access it through your institution.
1. Rheumatol Int. 2014 Sep 26. [Epub ahead of print]
The prevalence of systemic autoimmune rheumatic diseases in Canadian pediatric populations: administrative database estimates.
Shiff NJ(1), Lix LM, Joseph L, Duffy C, Tucker LB, Svenson LW, Belisle P, Bernatsky S.
Author information:
(1)Department of Paediatrics, Royal University Hospital, University of Saskatchewan, 103 Hospital Drive, Saskatoon, SK, S7N 0W8, Canada, natalie.shiff@usask.ca.
To estimate systemic autoimmune rheumatic disease (SARD) prevalence using administrative data for pediatric populations in four Canadian provinces. Physician billing claims and inpatient hospitalizations from Alberta, Manitoba,
Quebec, and Saskatchewan were used to define cases aged ≤18 years with a SARD diagnosis code in: one or more hospitalization, two or more physician visits within 2 years and at least 2 months apart, or one or more physician visit to a rheumatologist. Estimates ranged from 15.9/100,000 in Quebec [95 % confidence interval (95 % CI) 14.1, 18.0] to 23.0/100,000 in Manitoba (95 % CI 17.9, 29.2). SARDs were more common in females than in males across all provinces. There was a slightly higher prevalence among those living in urban compared to rural areas of Alberta (rate difference 14.4, 95 % CI 8.6, 20.1) and Saskatchewan (rate difference 13.8, 95 % CI 1.0, 26.6). Our results provide population-based
prevalence estimates of pediatric SARDs in four Canadian provinces.
PMID: 25257764 [PubMed - as supplied by publisher]
| Author: | Natalie Jane Shiff, Lisa M. Lix, Lawrence Joseph, Ciaran Duffy, Lori B. Tucker, Lawrence W. Svenson, Patrick Belisle , Sasha Bernatsky |
| Category: | Research Papers |
| Date: | November 12, 2014 |
This document is available on Google Books.
"Click here" to open a new browser tab and view this document.
| Author: | Craig Dickstein and Renu Gehring |
| Category: | Research Papers |
| Date: | October 8, 2014 |
Abstract
Background: Obesity is a pervasive problem and a popular subject of academic assessment. The ability to take advantage of existing data, such as administrative databases, to study obesity is appealing. The objective of our study was to assess the validity of obesity coding in an administrative database and compare the association between obesity and outcomes in an administrative database versus registry.
Methods: This study was conducted using a coronary catheterization registry and an administrative database (Discharge Abstract Database (DAD)). A Body Mass Index (BMI) ≥30 kg/m2 within the registry defined obesity. In the DAD obesity was defined by diagnosis codes E65 –E68 (ICD-10). The sensitivity, specificity, negative predictive value (NPV) and positive predictive value (PPV) of an obesity diagnosis in the DAD was determined using obesity diagnosis in the registry as the referent. The association between obesity and outcomes was assessed.
Results: The study population of 17380 subjects was largely male (68.8%) with a mean BMI of 27.0kg/m2. Obesity prevalence was lower in the DAD than registry (2.4% vs. 20.3%). A diagnosis of obesity in the DAD had a sensitivity 7.75%, specificity 98.98%, NPV 80.84% and PPV 65.94%. Obesity was associated with decreased risk of death or re-hospitalization, though non-significantly within the DAD. Obesity was significantly associated with an increased risk of cardiac procedure in both databases.
Conclusions: Overall, obesity was poorly coded in the DAD. However, when coded, it was coded accurately. Administrative databases are not an optimal datasource for obesity prevalence and incidence surveillance but could be used to define obese cohorts for follow-up.
| Author: | Billie-Jean Martin, Guanmin Chen, Michelle Graham, Hude Quan |
| Requirements: | PDF Reader |
| Category: | Research Papers |
| Date: | February 21, 2014 |
Download
ABSTRACT
Objective: We aimed to systematically review rheumatoid arthritis (RA) disease severity indices for use in administrative healthcare databases. We also provide an overview of alternative methods to control for RA disease severity in administrative database research.
Methods: We conducted a systematic review of studies that developed/validated an index for RA dis- ease severity using variables in administrative databases, and compared the convergent validity/reliability of the index with a standard measure of RA severity.
Results: After reviewing 539 articles, 2 studies were included. The claims-based index for RA severity (CIRAS) was developed in one study. Components of the CIRAS included tests for inflammatory markers, number of chemistry panels/platelet counts ordered, rheumatoid factor test, number of rehabilitation and rheumatology visits, and Felty’s syndrome. The CIRAS correlated moderately well with a previously validated RA medical records-based index of severity. The second study assessed whether current and lifetime treatment with disease-modifying anti-rheumatic drugs and/or biologics accurately predicted RA severity, as measured by the patient-reported Patient Activity Scale (PAS). Treatment variables did not fully distinguish patients in the highest and lowest quartiles of PAS scores (67.2% correctly classified).
Conclusion: Two claims-based indices of RA severity were identified but have some limitations for routine use. A concerted effort from experts in the field is needed to define, develop, and validate a widely applicable measure of RA disease severity for administrative database research.
(First Release Sept 15 2011; J Rheumatol 2011;38:2318–25; doi:10.3899/jrheum.110587)
| Category: | Research Papers |
| Date: | August 31, 2013 |
Abstract
There is growing interest in developing tools
and methods for the surveillance of chronic rheumatic diseases, using existing resources such as administrative health databases. To illustrate how this might work, we used
population-based administrative data to estimate and compare the prevalence of systemic autoimmune rheumatic
diseases (SARDs) across three Canadian provinces, assessing for regional differences and the effects of demographic factors. Cases of SARDs (systemic lupus erythematosus, scleroderma, primary Sjogren’s, polymyositis/dermatomyositis) were ascertained from provincialphysician billing and hospitalization data. We combined information from three case definitions, using hierarchical Bayesian latent class regression models that account for the
imperfect nature of each case definition. Using methods that
account for the imperfect nature of both billing and hospitalization databases, we estimated the over-all prevalence of SARDs to be approximately 2–3 cases per 1,000 residents. Stratified prevalence estimates suggested similar demographic trends across provinces (i.e. greater prevalence in females-versus-males, and in persons of older age). The prevalence in older females approached or exceeded 1 in100, which may reflect the high burden of primary Sjogren’s syndrome in this group. Adjusting for demographics, there was a greater prevalence in urban-versus-rural settings. In our work, prevalence estimates had good face validity and provided useful information about potential regional and demographic variations. Our results suggest that surveillance of some rheumatic diseases using administrative data may indeed be feasible. Our work highlights the usefulness of using multiple data sources, adjusting for the error in each.
| Category: | Research Papers |
| Date: | August 31, 2013 |
ABSTRACT
Objective. Little information exists regarding the economic burden related to inflammatory
myopathies. Our objective was to estimate health services costs in a large, unselected, population-
based sample of patients with inflammatory myopathies.
Methods.We identified subjects with polymyositis and dermatomyositis from administrative healthcare
databases (covering all beneficiaries, about 7.5 million) in Quebec province, Canada. Average
estimates of health services costs (physician visits, diagnostic tests and procedures, outpatient surgeries
and procedures, acute care hospitalizations) for 2003 were calculated by multiplying health
service use levels by the appropriate unit prices, determined from government fee schedules and
other sources. Multiple linear regression analyses were performed to establish whether specific factors
(age, sex, disease duration, region of residence, socioeconomic status, type of myositis, disease
severity) were associated with cost.
Results. We identified 1102 subjects with inflammatory myopathy from January 1, 1989, to January
1, 2003. About two-thirds were women (68.9%); average age at case ascertainment was 57.4 years
(SD 18.4). The average cost of all reimbursed health services, in 2008 Canadian dollars, was $4099
per patient (SD $9639). Costs increased with age, and were highest early in the disease course.
Greater disease severity (defined as the need for prior hospitalization for myositis) was also a strong
predictor of both physician costs and total costs.
Conclusion. These results indicate significant economic burden related to inflammatory
myopathies, with important demographic predictors. Our estimates suggest that the health services
costs in inflammatory myopathies may equal, or exceed, those of other serious diseases, such as
rheumatoid arthritis and systemic sclerosis.
(First Release March 1 2011; J Rheumatol 2011;
38:885–8; doi:10.3899/jrheum.101083)
| Category: | Research Papers |
| Date: | August 31, 2013 |
ABSTRACT
Objective. To examine the validity of case definitions for systemic autoimmune rheumatic diseases
[SARD; systemic lupus erythematosus (SLE), systemic sclerosis (SSc), myositis, Sjögren’s syndrome,
vasculitis, and polymyalgia rheumatica] based on administrative data, compared to rheumatology
records.
Methods. A list of rheumatic disease diagnoses was generated from population-based administrative
billing and hospitalization databases. Subjects who had been seen by an arthritis center rheumatologist
were identified, and the medical records reviewed.
Results. We found that 844 Nova Scotia residents had a diagnosis of one of the rheumatic diseases of
interest, based on administrative data, and had had ≥ 1 rheumatology assessment at a provincial arthritis
center. Charts were available on 824 subjects, some of whom had been identified in the administrative
database with > 1 diagnosis. Thus a total of 1136 diagnoses were available for verification against
clinical records. Of the 824 subjects, 680 (83%) had their administrative database diagnoses confirmed
on chart review. The majority of subjects who were “false-positive” for a given rheumatic disease on
administrative data had a true diagnosis of a similar rheumatic disease. Most sensitivity estimates for
specific administrative data-based case definitions were > 90%, although for SSc, the sensitivity was
80.5%. The specificity estimates were also > 90%, except for SLE, where the specificity was 72.5%.
Conclusion. Although health administrative data may be a valid resource, there are potential problems
regarding the specificity and sensitivity of case definitions, which should be kept in mind for future
studies.
(J Rheumatol First Release May 1 2011; doi:10.3899/jrheum.101149)
| Category: | Research Papers |
| Date: | August 31, 2013 |
Download
ABSTRACT
Objective: To determine how duration of observation affects estimation of incidence and prevalence of systemic lupus erythematosus (SLE).
Methods: SLE incidence and prevalence estimates from data periods as brief as 3 years (2001-2003) were compared to estimates from a 15-year period (1989-2003).
Results: The 15-year period incidence was 5.6/100,000 (95% CI 5.0–6.1) and the prevalence was 59.1/100,000 (95% CI 57.4–60.8). When a 3-year period was used, incidence was overestimated by 238.1% and prevalence underestimated by 66.0%.
Conclusion: SLE incidence and prevalence estimates vary considerably according to the observation period; more than 5 years of data is likely required.
(First Release June 15 2013; J Rheumatol 2013;40:1334–6; doi:10.3899/jrheum.121215)
| Author: | Ryan Ng, Sasha Bernatsky, Elham Rahme |
| Category: | Research Papers |
| Date: | August 31, 2013 |
Based on a presentation by Dr. Sasha Bernatsky at the BC Lupus Society Symposium held October 22, 2005.
Background
Dr. Bernatsky is currently an assistant professor in the Department of Medicine at McGill University. Her current focus is on understanding the risk of cancer in SLE. She is also co-editor of “Lupus: The Disease with a Thousand Faces.”
| Author: | Dr. Sasha Bernatsky |
| Category: | Research Papers |
| Date: | August 2, 2013 |
Lupus is a chronic autoimmune disease of limited awareness in Canada. It is not a rare disease and is estimated to affect more than 50,000 Canadians. Its symptoms mimic other illnesses, are varied and complex, and can strike any tissue or organ in the body. Common symptoms of lupus include skin rashes, sun sensitivity, joint pain, extreme fatigue, fever, chest pain and seizures.
Endorsed by the Canadian Medical Association, the recently released book, Lupus, The Disease with a Thousand Faces is a very important resource for people living with lupus, especially due to a limited availability of Canadian information about the disease. Lupus Canada worked in partnership with publisher, Key Porter Books, Dr. Jean-Luc Senécal and Dr. Sasha Bernatsky to fulfill the need for a widely available, up to date guide for those diagnosed with lupus, their caregivers, friends and families.
| Author: | Lupus Canada, Dr. Jean-Luc Senécal and Dr. Sasha Bernatsky |
| Category: | Research Papers |
| Date: | December 3, 2012 |
RA and its impact on the workplace - How to lessen the cost burden of rheumatoid arthritis.
| Author: | Dr. Diane Lacaille |
| Category: | Research Papers |
| Date: | July 4, 2011 |


 Research Papers Archive
Research Papers Archive

















-155x200.jpg)