This section includes public consensus statements on the use of administrative health data in rheumatic disease: research and surveillance.
Number of files in this category: (8)Download
Abstract
Routinely collected health data, obtained for administrative and clinical purposes without specific a priori research goals, are increasingly used for research. The rapid evolution and availability of these data have revealed issues not addressed by existing reporting guidelines, such as Strengthening the Reporting of Observational Studies in Epidemiology (STROBE). The REporting of studies Conducted using Observational Routinely collected health Data (RECORD) statement was created to fill these gaps. RECORD was created as an extension to the STROBE statement to address reporting items specific to observational studies using routinely collected health data. RECORD consists of a checklist of 13 items related to the title, abstract, introduction, methods, results, and discussion section of articles, and other information required for inclusion in such research reports. This document contains the checklist and explanatory and elaboration information to enhance the use of the checklist. Examples of good reporting for each RECORD checklist item are also included herein. This document, as well as the accompanying website and message board (http://www.record-statement.org), will enhance the implementation and understanding of RECORD. Through implementation of RECORD, authors, journals editors, and peer reviewers can encourage transparency of research reporting.
| Author: | Eric I. Benchimol, Liam Smeeth, Astrid Guttmann, Katie Harron, David Moher, Irene Petersen, Henrik T. Sørensen, Erik von Elm, Sinéad M. Langan, RECORD Working Committee |
| Category: | Consensus Statements and Best Practices |
| Date: | November 30, 2015 |
The Health Analyst’s Toolkit is specifically designed for analysts and epidemiologists working in, or for, Ontario’s Local Health Integration Networks (LHINs). The toolkit has two sections; the Knowledge section covers a variety of important topics relevant to LHIN analyses, while the Data section identifies and describes highly relevant data sources.
| Author: | Sten Ardal, Linda Baigent, Nam Bains, Carley Hay, Paul Lee, Stephanie Loomer |
| Category: | Consensus Statements and Best Practices |
| Date: | September 24, 2015 |
Background
The 2011 Health Analyst’s Toolkit is an updated, expanded version of the original toolkit that was designed in 2006 for analysts working in, or for, Ontario’s Local Health Integration Networks (LHINs). That version, like this one, was intended for use by people who had some data analysis experience and familiarity with basic technical language and concepts. The creation of the LHINs in 2005 had led to the need for an understanding of new geographic levels of analysis in Ontario. Because LHIN boundaries differ from historical geographies, there was considerable demand for recalculation and for new analyses that would conform to the LHIN boundaries. In January 2006, the Health Analyst’s Toolkit was created to support analysts to meet this demand. The toolkit was divided into two sections, Knowledge and Data, with much of the former devoted to a variety of topics relevant to LHIN-level analyses. For the Data section, contributors identified and described highly relevant data sources that would support the needs of LHIN analysts. All contributors to the original toolkit had experience manipulating data to provide local area estimates, health status measures, and healthcare utilization indicators. When asked to describe a resource guide that would inform their own work, the content they identified is that which is covered in the toolkit.
| Author: | Health Analytics Branch |
| Category: | Consensus Statements and Best Practices |
| Date: | September 24, 2015 |
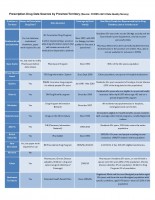
Prescription Drug Data Sources by Province/Territory (Source: CCDSS v2013 Data Quality Survey)
| Author: | Source: CCDSS v2013 Data Quality Survey |
| Category: | Consensus Statements and Best Practices |
| Date: | September 16, 2015 |
Download
This manual presents the lists of causes of death for tabulation and dissemination of mortality data by the National Center for Health Statistics (NCHS) under the Tenth Revision of the International Classification of Diseases (ICD-10) recommended by the World Health Organization (WHO)(1). NCHS began using this manual for data year 1999. A total of eight (8) tabulation lists are described. Seven (7) of the lists are used for both underlying and multiple causes of death, and one for multiple causes of death only. The tabulation lists in this report have been developed to maintain continuity with the lists from the Ninth Revision of the International Classification of Diseases (ICD-9)(2); to facilitate trend analysis; and to separately identify causes of death that are of public health and medical importance. Periodically, NCHS Instruction Manuals are updated in order to correct errors and to reflect needed modifications dictated by changes in medical science, statistical practice and processing technology. This update represents the fourth to the lists that are used by NCHS to present mortality data and include the addition of ICD-10 codes for Terrorism Deaths for data year 2001 and WHO updates to ICD-10 for data year 2003. States and other federal agencies and organizations using the NCHS lists should incorporate these changes as soon as possible. All changes listed in the “Notices of Change” have been incorporated into this updated version of the manual (see Appendices A-D).
| Author: | Robert N. Anderson, James A Weed, Kenneth D Kochaneck, LloydReich, Brenda Gillum, Jeffrey D. Maurer, Linda Le |
| Category: | Consensus Statements and Best Practices |
| Date: | September 9, 2015 |
| Author: | CANRAD Network |
| Category: | Consensus Statements and Best Practices |
| Date: | July 23, 2013 |
Consensus statements for best practices when using administrative data for rheumatic disease research and surveillance.
CANRAD Network Authors: D. Lacaille, L. Lix, C. Bombardier, S. Bernatsky, J. Askling, A. Aviña-Zubieta, E. Badley , C. Barber, C. Barnabe, S. Benseler, L. Bergeron, L. Bessette, B. Bobechko, C. Cooke, J. Curtis, W. Dixon, C. Duffy, S. Edworthy, B. Elias, D. Feldman , P. Fortin, J. Hanly, G. Hawker, J. Henderson, M. Hudson, S. Jean, J. Kopec, B. Kuriya, J. Labreque, A. Leeong, D. Levy, S. Lim C. Mackay, P. McCrea, P. Nestman, K. Oen, M. Patterson, C. Peschken, D. Power, E. Rahme, A. Rosenberg, L. Rochette, N. Shiff , E. Silverman, C. Sirois, M. Smith, D. Solomon, G. Soon, E. Stringer, S. Suissa, L. Svenson, L. Tucker, E. Vinet, J. Widdifield.
| Author: | CANRAD Network |
| Category: | Consensus Statements and Best Practices |
| Date: | July 23, 2013 |
Consensus Statements for The Use Of Administrative Health Data In Rheumatic Disease: Research And Surveillance
| Author: | Sasha Bernatsky, MD, PhD1,2, Lisa Lix, PhD3, Siobhan O'Donnell, MSc4, Diane Lacaille, MD MHSc5, and the CANRAD Network |
| Requirements: | PDF Reader |
| Category: | Consensus Statements and Best Practices |
| Date: | November 2, 2012 |


 Consensus Statements & Best Practices Archive
Consensus Statements & Best Practices Archive




-200x186.jpg)
